Nuclear fission
Every matter in the world is made up of atoms and an atom consists of a nucleus and the surrounding electrons. The basic fuel for nuclear reaction is derived from natural uranium, a very heavy metal. Natural uranium is composed of 3 isotopes: uranium-235 (0.71% by content), uranium-238 (99.28% by content) and a minute portion of uranium-234. Nuclear fission is initiated when the nucleus of a uranium atom is hit by a neutron and split into two halves. In addition to splitting the nucleus into two halves, the process releases two or more neutrons and a large amount of energy in the form of heat. The released neutrons in turn hit more uranium nuclei and release more neutrons to produce a chain reaction. The fission process produces a large amount of energy for power generation.
The high energy neutrons released by fission travel at very high speeds (fast neutrons) and must be slowed down to increase its probability of hitting more uranium atoms and in turn induce more fission in the reactor. Commercial type nuclear reactors normally use a moderator to slow down the high energy neutrons to low energy neutrons (thermal neutrons). Ordinary water, graphite and the more expensive heavy water are used as moderators for various types of commercial nuclear reactors.
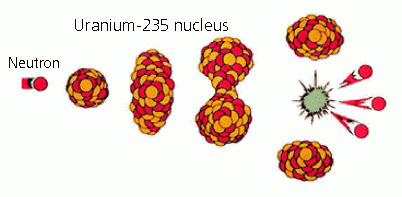
Nuclear fission and chain reaction